IN THE MICROSCOPIC ARENA, A RUTHLESS KILLER LURKS
It’s almost hard to fathom that the most prolific killer on planet Earth is a microscopic speck far tinier than the smallest bacteria. Yet viruses that infect and destroy bacteria, known as bacteriophages or simply phages, are Earth’s most abundant organism with an estimated 10³¹ in number globally. This deadliest entity on Earth are ruthless killers of their prey.
To put that into perspective, there are more phages on Earth than every grain of sand across all the world’s beaches and deserts combined. And these tiny assassins are continuously driving evolution through an endless biological arms race with their prey: bacteria.
“The concept is that the enemy of my enemy is my friend,” explains Dr. Lillian Musila, an infectious disease researcher at the Kenya Medical Research Institute (KEMRI) in Nairobi. She and teams of researchers across Africa have turned to phages as potential weapons against one of humanity’s most feared microbial nemeses – superbugs.

Photograph by Jess Craig
WHEN ANTIBIOTICS FAIL, PHAGE HUNTERS RESPOND
Since the 1940s, antibiotics have been the undisputed champions saving innumerable lives from bacterial diseases. Yet over decades of use and misuse, many types of disease-causing bacteria have evolved ways to evade antibiotics through antimicrobial resistance (AMR).
The World Health Organization now considers AMR to be one of the most critical threats to global public health and medicine. It’s estimated that superbug infections directly caused over 1 million deaths worldwide in 2019 alone. Without new drugs to fight ever-resistant bacteria, medicine faces a grim return to the pre-antibiotic era. This is where the deadliest entity can come into play.
“It felt like we were just going around declaring doom and the impending end of the world,” recalls Musila. After seeing many Kenyan patients infected with bacteria resistant to all known antibiotics, she refused to just document the crisis. Musila sought solutions – and found promise in humble phages.
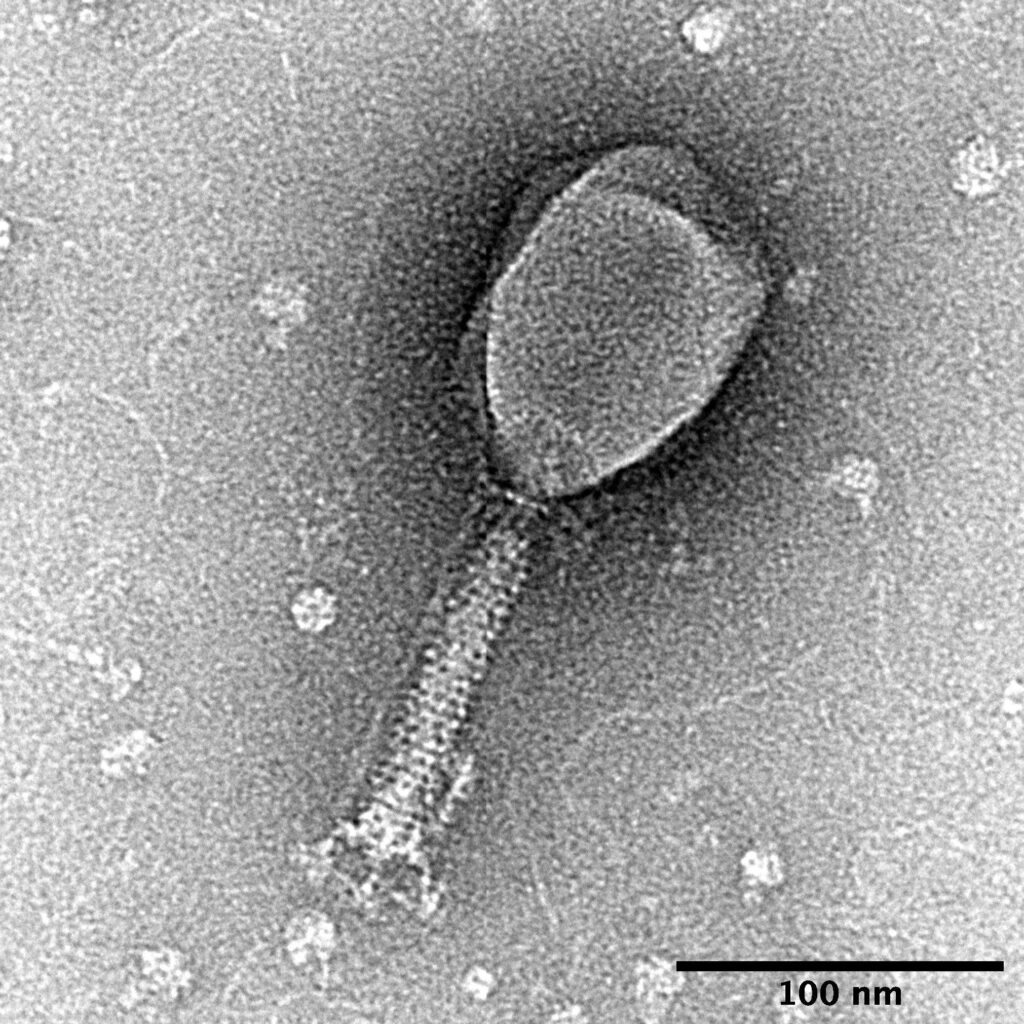
![Structural model at atomic resolution of bacteriophage T4[1]](https://factbrainiac.com/wp-content/uploads/2024/03/Structural-model-at-atomic-resolution-of-bacteriophage-T41.jpg)
Phages occur abundantly anywhere bacteria thrive and die, especially in sewage and polluted wastewaters. So in 2016, Musila recruited research assistants Moses Gachoya and Martin Georges for a novel mission: hunting for phages in Nairobi’s waste systems that could kill those antibiotic-resistant superbugs plaguing Kenyan hospitals.
The pair collected sewage samples, filtered out microbes like Salmonella and E. coli to feed potential phages, then unleashed those viruses on Petri dishes swarmed with bacteria. Where viral assassins did their work, clean circles emerged in the bacterial growth showing precise targets slain. Promising annihilators were then isolated, sequenced, and banked by Musila’s team for future study on difficult bacterial infections.
A NATURAL THERAPY
Over six years, the lab has gathered a menagerie of over 150 phages able to defeat common superbug strains like Klebsiella pneumoniae and Pseudomonas aeruginosa. Their efforts even attracted funding from the U.S. Department of Defense to keep exploring phages against the toughest military foe: drug-resistant infections.
And the KEMRI team is far from alone in exploiting phages as allies, both across the African continent and globally. With AMR forecast to cause a GDP drop between 1.1% and 3.8% globally by 2050, governments and organizations are finally turning to viruses as natural therapy.
A CYCLE OF EVOLUTION DRIVES DIVERSITY – AND BETTER MEDICINE
The key to the deadliest entity, phages’ success against bacteria where antibiotics fail is co-evolution. These mortal enemies have constantly adapted against each other for eons through a biological arms race without parallel. Wherever they compete across the planet, this cycle of crisis and response has birthed astonishing diversity in both species.
Bacteria develop new defenses like surface proteins to evade phages, then phages evolve new attachment structures to target those proteins and resume their feast. Rinse and repeat endlessly through geological ages. It’s a microcosmic mirror to the dynamics Charles Darwin witnessed between predators and prey driving natural selection.
Yet such evolutionary force matched with staggering numerical advantage has lent phages unique strengths bacteria cannot escape for long. Using sophisticated nanoscale equipment like tails, hooks, and chemical receptors, phages keep finding ways through bacteria’s toughest barriers.
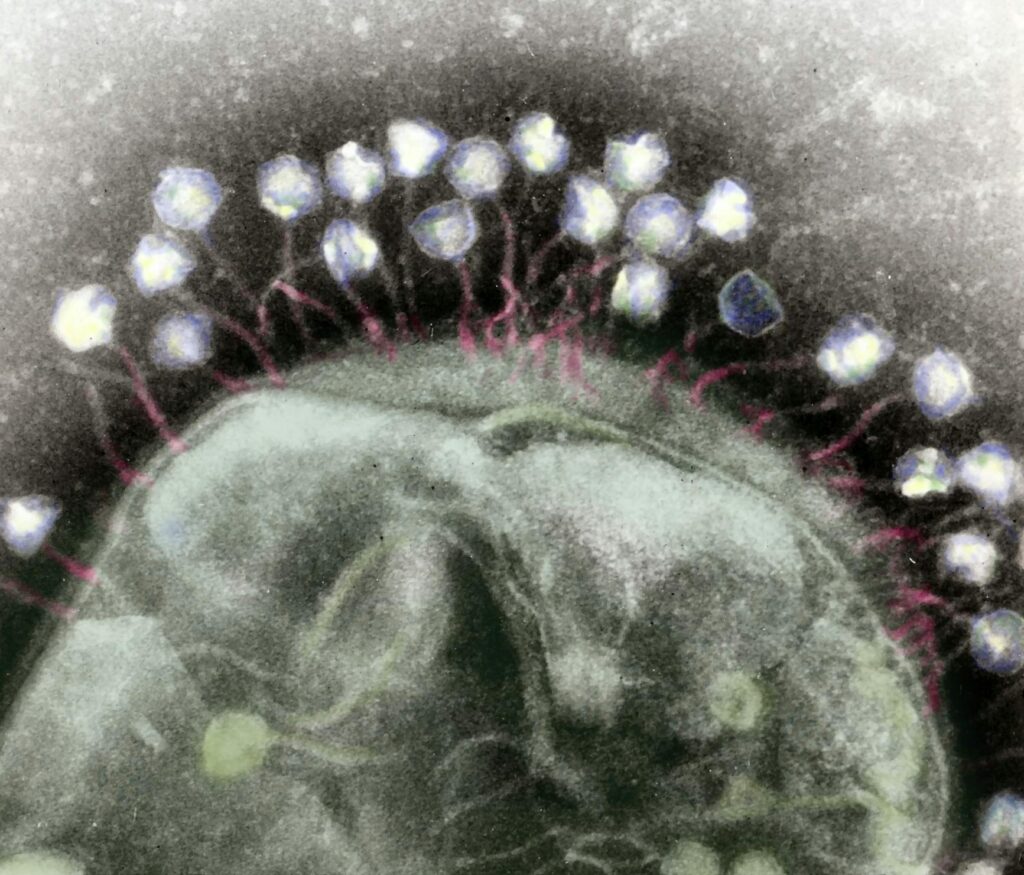
bacteriophages attached to a bacterial cell wall
(Credit: Wikimedia Commons)
HOW THE DEADLIEST VIRUS WORKS
Once inside a bacterial cell, a phage converts the host into a viral replication factory until eventually the mechanism fails and underlying structure bursts from sheer numbers of newly copied phages escaping like swarming locusts to invade other cells. Killing never ceases. Much like the iconic creature in the Alien films, no earthly microbe has proven truly immune.
Additionally, because phages continually evolve naturally against local bacteria where they live through this lifecycle, they tend to make the most lethal and targeted anti-microbials compared to traditional antibiotics when isolated from the same environment. Rather than blast all bacteria equally, phages specifically infect only strains with matching defense profiles. This razor focus prevents damage to beneficial bacteria living symbiotically in the body and environment.
Thus a key tenet emerges in the battle against superbugs: to defeat a mighty foe, recruit their most ancient enemy with endless brutality and evolving tools to match. Within Earth’s realm of microscopic organisms, none fits that role like the phage.
HUNTING VIRUSES TO BEAT REGIONAL SUPERBUGS
Phage therapy faced a brief setback last century with the advent of mass manufactured antibiotics. Today however, drug-resistant infections have granted phages a triumphant medical return in regions where superbugs hit hardest. The simplicity of finding environmentally well-adapted phages has enabled developing countries burdened by AMR to leapfrog ahead with novel solutions.
“It’s an accessible technology for the developing world. I think that’s the beauty of it,” says Dr. Musila. But local isolation is key. Her team in Nairobi could easily grow bacteria shipped from Russia. Yet, these viruses from Russian soil proved far less effective at killing Kenyan bacterial strains compared to phages gathered locally.
This principle led Dr. Ivy Mutai of the Institute of Primate Research and colleagues to begin capturing Nairobi’s phages better evolved against regional superbugs. At Uganda’s Makerere University, Dr. Jesca Nakavuma found phages that target multi-drug resistant E. coli. Dr. Nakavuma uses them to save fish from a common tank-based bacteria. In Malawi and across sub-Saharan Africa, lack of access to existing antibiotics already cruelly shows how phage solutions can succeed where prohibitive costs have failed communities.
A NEW DAWN IN DISCOVERY
The accessible nature of phage bio-prospecting has even launched a new generation of young African researchers against infectious diseases. These researchers work via grassroots NGOs like Phages for Global Health. Many developed nations now recruit graduate students into massive phage discovery networks. Most notable is the Graham Hatfull’s SEA-PHAGES initiative through the University of Pittsburgh. Nearly 40,000 pupils have passed through his program to add over 10,000 new phages to the scientific vaults since 2008.
Hatfull gets requests to supply phages for emergency clinical use in the U.S. around every 48 hours now. Those life-saving shipments owe their origins to students spending just a few weeks hunting viruses in backyards and barnyards. Phage therapy has become public health’s best example of everyday people in any country contributing to global medical solutions.
Of course, challenges still loom around characterizing phages for safety and potency compared to traditional drugs, or the ability for bacteria to also evolve resistance. Yet for each setback against a particular phage, countless unseen viral rivals lurk nearby awaiting fresh exploitation. While not a permanent solution, phages offer more durable adaptive attacks on superbugs through affordable routine isolation compared to chemical antibiotics.
In many ways, the world’s deadliest entity rests underfoot anywhere water flows. Trillions of tiny natural assassins primed by aeons of evolution against resistant prey. An invisible army to aid humanity against its oldest microscopic foe while driving forward essential biodiversity for global health. To save future millions, disease-fighters worldwide now unleash Earth’s greatest serial killers upon itself.
So minuscule, yet so almighty through chemistry and chance. The mighty bacteriophage has arrived as medicine’s new apex predator. No microbe survives unchecked forever once marked for consumption by this, the apex killer in life’s microbial jungle.



